
© Claudio Caridi / Adobe Stock
How We Can Become Spiderman
Will it be possible for “normal” people to walk on walls and ceilings in the future?
Every single person has probably imagined once what life as a superhero with special skills would be like. Of course, we all know that most of the skills that actually define superheroes are supernatural and (unfortunately) come entirely from the imaginations of the authors and filmmakers.
Most of the skills? One of the most famous superheroes is Spiderman, who notoriously manages to climb up supposedly smooth walls in comics and films. If you take a look at the world of animals, it is easy to see that it is not only spiders but many animals that move across walls and ceilings much like Spiderman.
The superhero skills of Spiderman are thus less supernatural than initially thought and one could ask the question: Will it be possible for us “normal” people to walk on walls and ceilings in the future? And if not, what would a Spiderman suit have to be like so that fiction turns into reality? Bugs, spiders, geckos, and co. provide the answers.
Why are geckos able to walk on walls? First of all, let us try to understand the mechanism that enables geckos, spiders, and bugs to move along walls despite their own body mass. This type of movement is made possible by the comparably large contact area between the animals’ feet and the wall. Figure 1 shows the situation for several insects – under a microscope, so-called setae (the Latin word for bristles) on the feet of the animals become visible. They then split up into several smaller hairs – so-called spatulae. The bigger the animal, the smaller the spatulae and thus the bigger the contact area between the wall and the animal. The bond strength, also known as adhesion, that allows for insects but also other animals, such as geckos, to crawl over a wall is initiated by the interaction between the feet and the contact surface.
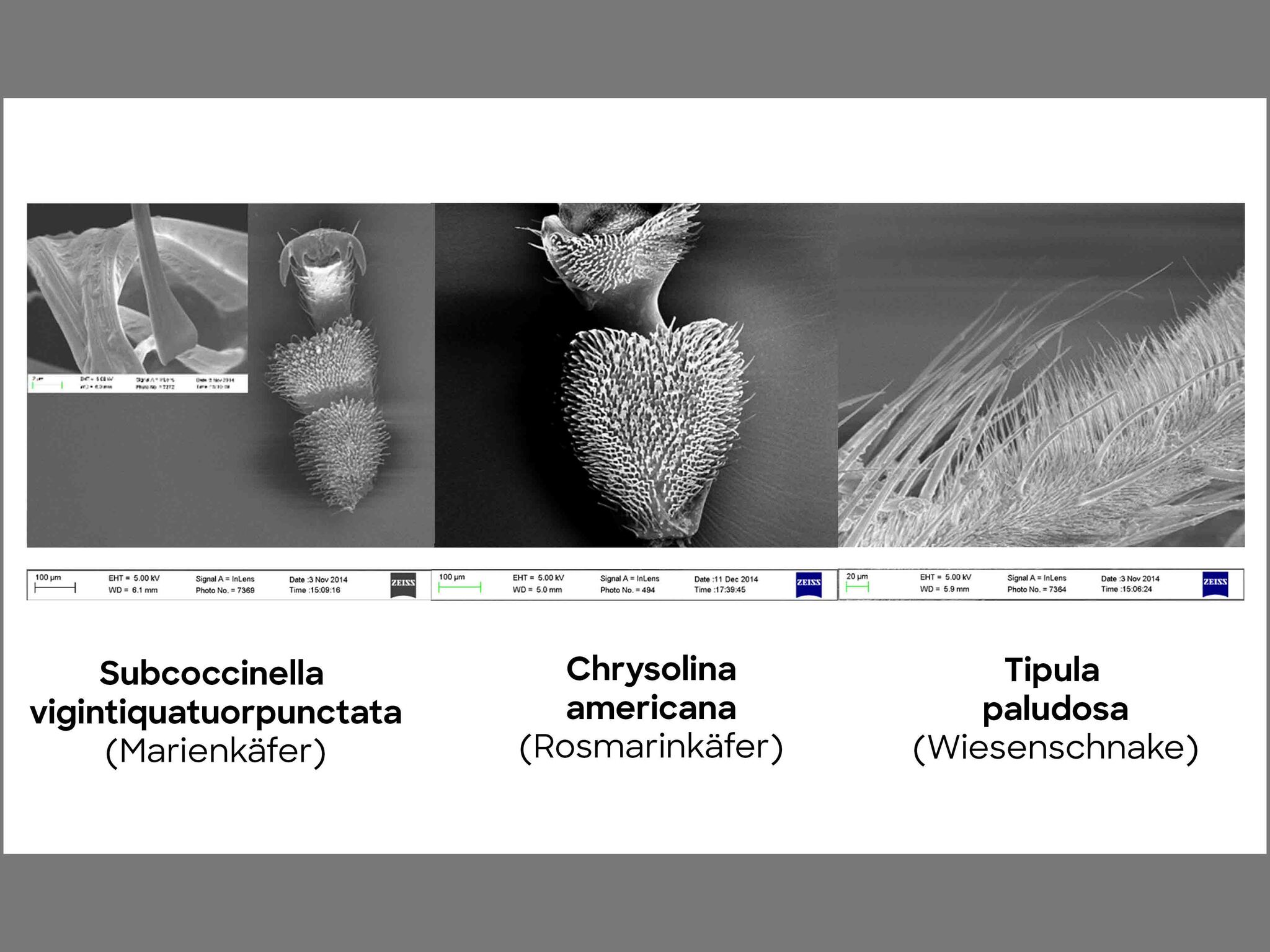
© David Labonte, Department of Bioengineering, Imperial College London, UK
In order to understand this, we do however need an idea of what gecko feet and walls are made up of. Let’s imagine that we enlarge the gecko foot and the wall by a large factor using a magnifying glass. If the magnification is sufficient, we can determine the atoms that make up the foot and wall. An atom is a type of building block of the material and we often visualize it as a very small sphere that has a collection of even smaller spheres in its core as well as randomly moving, even smaller spheres in the rest of the atom. The moving spheres carry negative electric charge and are called electrons. Figure 2 shows our model visualization.
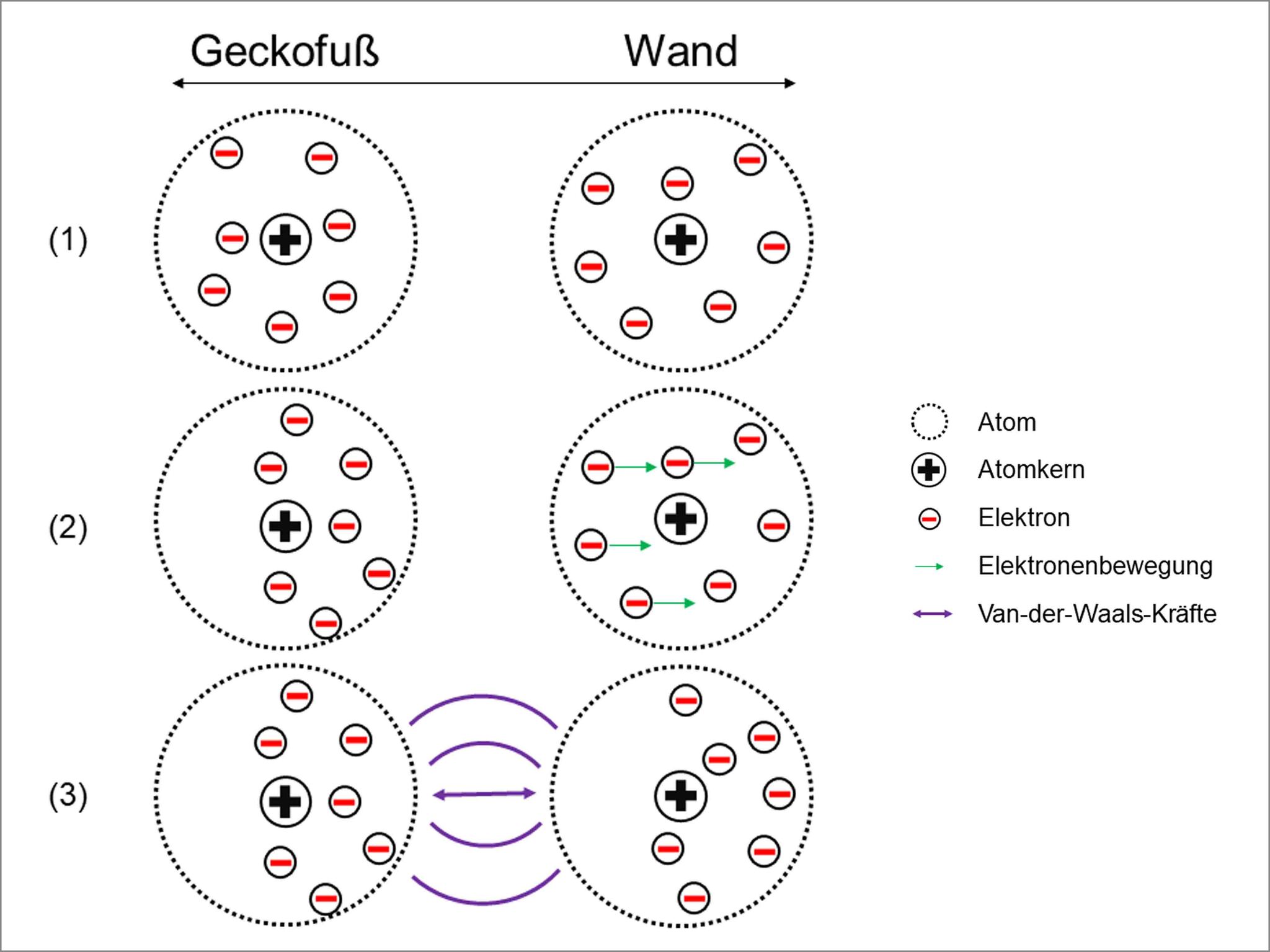
© Universität Duisburg Essen
The illustration explains the following: Two closely located atoms (1) – let’s say one is from a gecko foot and the other from a wall – influence each other like magnets. For example, for a short moment there will be more electrons on the one side of an atom than on the other side. The side with more electrons thus carries a negative electrical charge for a short period and ensures that electrons in the other atom temporarily move as far away from the negative pole as possible (2). This happens based on the basic theory that same poles, or rather charges, repel each other.
As the side of the one atom with less electrons consequentially appears to carry a more positive charge than its counterpart, both one negatively and one positively charged atom side are adjacent. As a result, the atoms are attracted to each other, much like the north and south poles of two magnets (3). This adhesion force is created everywhere where the gecko foot is extremely close to the wall.
With figure 1, we are able to connect the anatomy of the setae (bristles) and spatulae (small hairs) to the size of the animal. A bigger number of spatulae increases the contact surface with the base surface, which in turn creates more interaction between the gecko foot and the wall. Generally, a high bond force is created as the gecko needs it to hold its own weight. The adhesive force that is created by the interaction between atoms is the sum of the many attractions between gecko foot atoms and wall atoms, which is also called the Van der Waals force.
Why Will Evolution Disappoint Us in the Short Term?
We only know the physical reasons for why small reptiles, amphibians, and insects can apparently walk on walls despite their own weight. Why did Mother Nature not equip us and our feet with the structures in figure 1?
The bigger the size of the living creature, the higher the required proportion of contact surface to the wall.
The problem is down to the fact that increasingly less body surface stands in relation to the body volume as soon as the body grows. As described, surface, or rather contact surface between the body and wall is, however, essential for the creation of bond forces. More surface is required the bigger and heavier the body is. In comparison, geckos need 200% more of their body surface to stick to walls than mites do. Figure 3 compares different animal sizes and the proportional percentage of body surface that is equipped with bond structures.
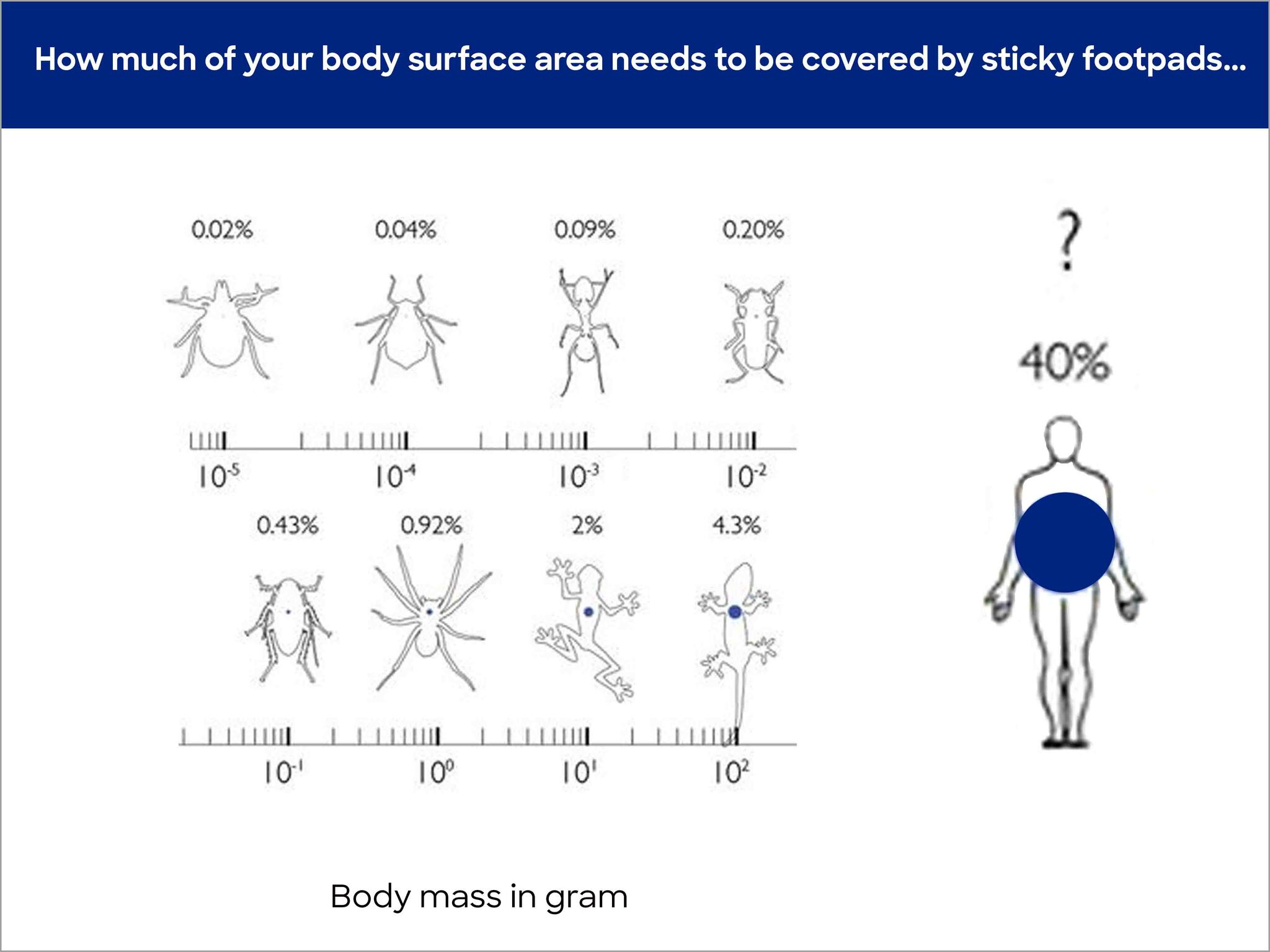
© David Labonte, Department of Bioengineering, Imperial College London, UK
If we transfer this calculation to people, crazy numbers result. In order to enable a 1.8 m high and 80 kg heavy man to be able to walk on walls, 40% or 0.8 m2 of his skin would have to be located on the soles of his feet (source: wikipedia). This would correspond to European shoe size 145. To compare: The biggest person ever (2.72 m) had an EU shoe size of 76, which is roughly the length of a forearm including an extended hand. He made it into the Guinness Book of Records with that shoe size.
The necessary anatomical changes to the human body would be too severe in the middle term and the development of such huge feet with bond structures would rather be an obstacle and impractical for daily life. Is it possible to remedy the situation in another way?
The Scientific Answer
Researchers developed the idea of retrofitting the bond structures from geckos’ feet onto humans artificially. The bionic climbing aids must not only be similar to the setae and spatulae from the animal kingdom in order to create the required surface contact to the base, but must also fulfil a whole set of other requirements. Firstly, they must be water and dirt repellent in order to guarantee adhesion to the wall. The climbing aids must also be made of a sufficiently flexible material that fits to suit the uneven areas and roughness of surfaces (source: Pugno, N. M. (2008), Spiderman gloves, Nano Today, Volume 3, Seiten 35-41).
At the same time, the material must be very stable in order to withstand the strains. One aspect that is very important is that the climbing aids must be able to come away from the base surface easily. However, a bond must be maintained upon there being slight movement so that a release at the wrong moment is avoided.
Carbon nanotubes combine the described qualities and are theoretically suitable as a replacement for setae and spatulae (figure 1) from the animal kingdom. As the name already says, the carbon nanotubes are made of carbon and form tubes that are only a few nanometers thick. In comparison to steel, they are 100 times more stable with only 1/6 of the weight (source: fuelcellstore.com).
However, research is also being carried out on column-shaped structures made of silicone elastomers that can be seen in figure 4. The illustration shows a microstructure adhesion surface that allows for the reversible, residue-free bonding of objects. The structure allows for the efficient usage of the Van der Waals interaction for reversible / controllable adhesion.
A nearly 100 kg man was able to climb up an eight meter-high, smooth glass surface with the help of nano climbing aids on his hands.
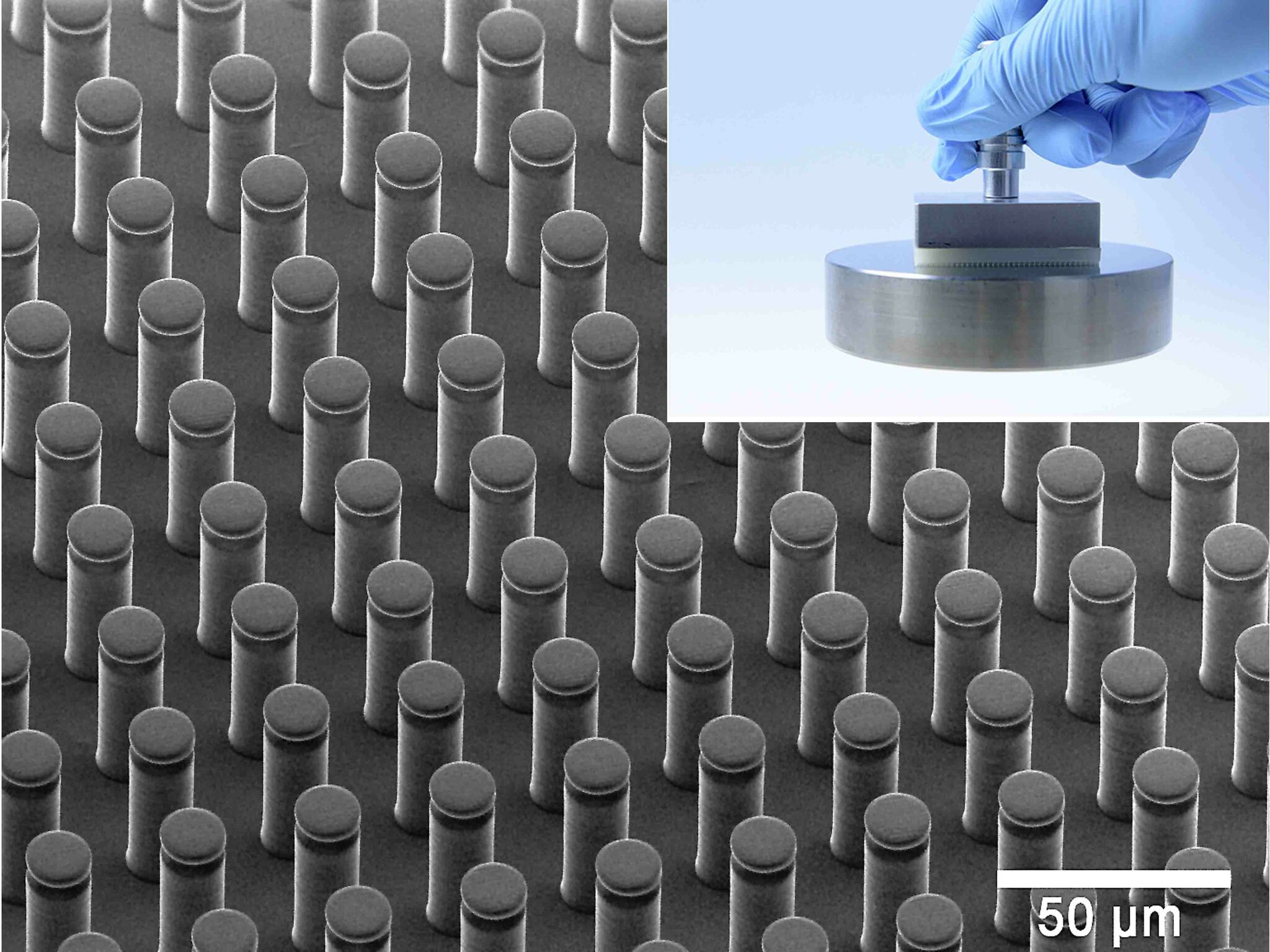
© René Hensel, INM – Leibniz-Institut für Neue Materialien
Whether there will be a type of commercial Spiderman suit in the in future remains to be seen. Researchers from the US Ministry of Defense have already shown that the approach is generally promising. A nearly 100 kg man was able to climb up an eight meter-high, smooth glass surface with the help of nano climbing aids on his hands.
It also remains to be seen which practical applications bionic climbing aids may have. Maybe in the not too distant future, we will see window cleaners who are trying their luck as hobby crime-fighters.
Have We Piqued Your Interest?
Detailed content on the topic of adhesion is taught in the field of mechanical process engineering, for example in the aerosol and nano-technology and particle technology lectures, at the University of Bremen. The lectures do not, however, focus on the Spiderman suit of the future but rather on aerosols, nanoparticles, other particle systems, and technical processes in which these occur.
This article comes from Science Blog
The MINTScience Blog (“MINT” is the German term for “STEM”) from the University of Bremen explains complex research topics to a broad audience. Students and early-career researchers wish to use the blog to clearly explain difficult issues. At the beginning of 2021, the editorial team celebrated success in the MINTchallenge. The team was able to win over the jury of the competition held by the Stifterverband and placed 3rd.